

RELIANCE project develops an entirely new class of biocidal additive. The antimicrobial action of the Cu-SMIN nanoparticles is enhanced due to the contact-killing antimicrobial properties provided by non-ionic copper on their structure, combined with the antimicrobial activity of encapsulated essential oils (EOs). Essential oils are natural, eco-friendly, safe and easily biodegradable agents that have been reported to be effective against bacteria, fungi and viruses in certain concentrations. Due to their multicomponent nature, their antimicrobial activity is not attributed to a specific mechanism but is instead, the result of the action on multiple targets in the cells. For this reason, their application does not lead to bacterial resistance and they appear to be suitable to fight multi-drug resistant bacteria.
To establish the antimicrobial activity of the selected essential oils in RELIANCE, namely thymol, eugenol, carvacrol, and menthol, the initial research phase of the project involves rigorous testing using laboratory-based techniques and procedures. This phase is important for understanding the effectiveness of the selected essential oils on the inhibition of the microorganisms’ growth.
ISO Method for antibacterial activity on the surface (ISO 22196):
ISO Method for Viruses:
Antimicrobial susceptibility testing for viruses, especially when it comes to antiviral drugs, is a more specialized area and may not follow ISO standards in the same way as bacteria. The methods for assessing antiviral susceptibility often vary depending on the virus, the drug being tested, and the laboratory's capabilities. However, some general principles are followed:
It's essential to note that specific methodologies for virus susceptibility testing can vary widely, depending on the virus in question and the available laboratory resources. While ISO standards may provide some guidance for quality control and best practices, the exact protocols often depend on the unique characteristics of each virus and drug combination.
Results
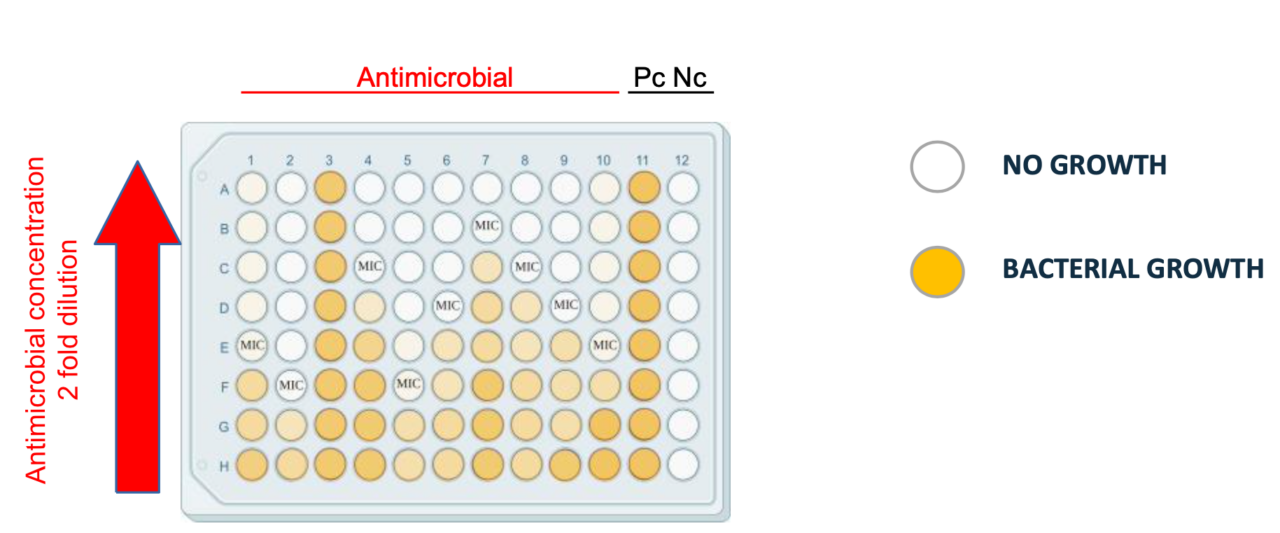
Dilution tests are central in assessing the efficacy of antimicrobial agents against microorganisms. In these tests, microorganisms are exposed to a range of dilutions of the antimicrobial agent in a broth. The lowest concentration of the antimicrobial agent that, under specific laboratory conditions, prevents visible growth of the microorganisms within a defined timeframe is referred to as the Minimum Inhibitory Concentration (MIC). The MIC serves as a quantitative measure, assisting clinicians in determining the susceptibility of the microorganism to the antimicrobial agent and guiding treatment decisions. It is crucial to maintain strict control and standardization to ensure reproducibility of results within and between laboratories, as variations can significantly affect outcomes.
With these assumptions, the antimicrobial capacity of essential oils is tested and preliminary tests indicate that Carvacrol exhibits higher inhibitory activity compared to Eugenol. Additionally, it is observed that E. coli is more sensitive to the tested essential oils than B. clausii.
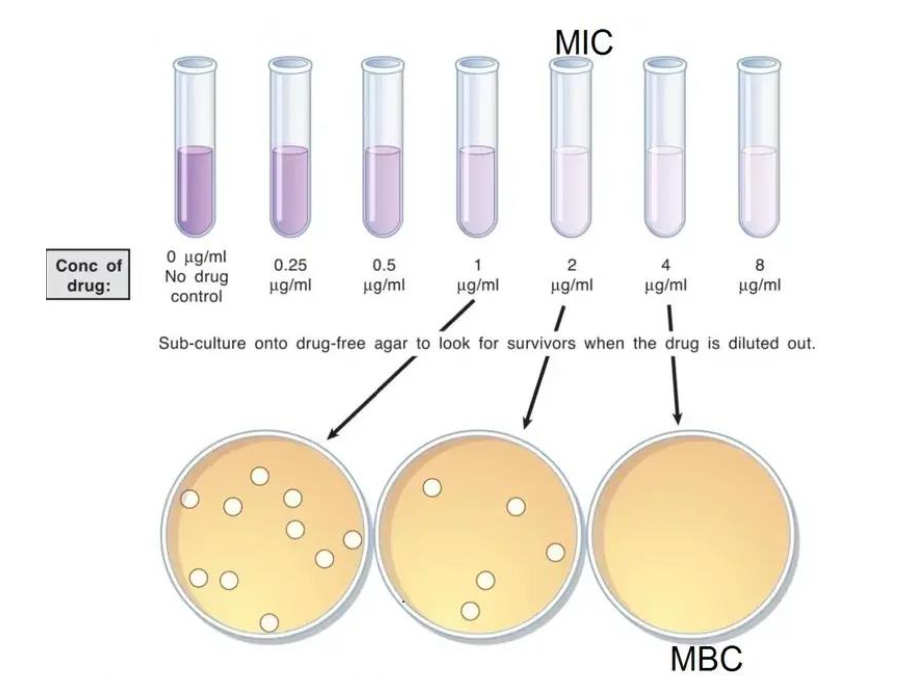
The Minimum Bactericidal Concentration (MBC) is determined by subculturing the broths used for MIC determination onto fresh agar plates. MBC represents the lowest concentration of the antimicrobial agent that results in the death of 99.9% of the bacteria being tested. For both Eugenol and Carvacrol, the MBC is found to be 1-2 dilutions higher than the MIC, indicating that a slightly higher concentration is needed to achieve bacterial killing.
Notably, after just 5 minutes of incubation, the results show an impressive 98% inhibition of bacterial growth. At a concentration of 0.6%, both essential oils successfully eliminate 100% of E. coli, S. aureus, and B. clausii within just one minute. Interestingly, Thymol demonstrates that S. aureus is more susceptible to its inhibitory effects compared to E. coli. This rapid and effective inhibition demonstrates the potency of the tested essential oils against the microorganisms.
The virucidal activity of the oils was assessed by plaque assay. The virus, incubated with an established concentration of the oils = 0,5%, was ten-fold diluted and inoculated in a cell monolayer for 1 hour. Then, cells were cultured with a semisolid overlay for several days and finally washed and stained using Crystal violet to visualize and count plaques forming units.
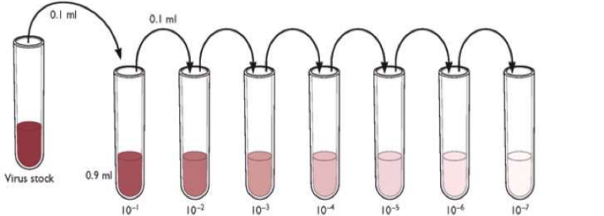
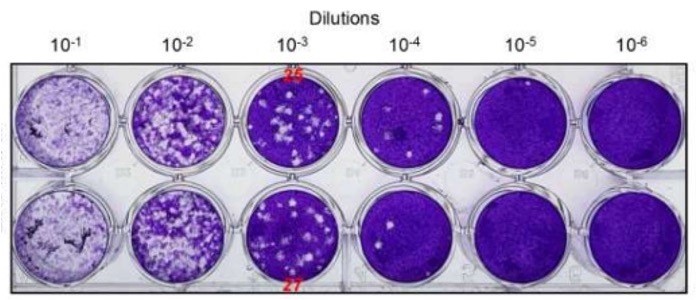
Different results have been obtained, depending both on the oil and the virus tested. Moreover, it has been observed that the number of infectious particles incubated with the oils decreases in a time-dependent manner. Preliminary tests showed that Carvacrol and Eugenol diluted to 0.5%, were able to inhibit CHIKV in a few minutes of contact: virus survival was 58.9% after 1 minute of treatment with Carvacrol and 53.1% after 3 minutes of treatment with Eugenol. Similar percentages have been observed also against SARS-CoV-2. Eugenol has been tested also against the pandemic influenza virus H1N1 /09 showing a two-log reduction (98% inhibition), after 5 minutes of incubation.
Bacteriophage MS2, a model for non-enveloped viruses, has been incubated at different times with Eugenol and Carvacrol. The oils have been diluted to a final 0,5% concentration as for all the experiments described above, but some virucidal effect has been observed even at 60 minutes of incubation. On the contrary, Eugenol to a 1% final concentration, showed a two-log reduction after 15-30 minutes exposure.
In conclusion, the obtained results confirm those available in the literature: essential oils are more efficient against enveloped viruses than non-enveloped ones.
These findings provide valuable insights into the potential use of essential oils as antimicrobial agents and highlight their effectiveness in inhibiting bacterial growth.
Looking to the Future
The next work activities will extend to deliver smartphone-assisted electrochemical paper-based devices for on-site detection of antimicrobial surface effectiveness.
Contributor: University of Rome Tor Vergata & ISBD




Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Health and Digital Executive Agency (HADEA). Neither the European Union nor the granting authority can be held responsible for them.
© All Rights Reserved 2022 | Reliance-HE
Designed and Developed by Europroject