Characterizing the antimicrobial activity of bioactive compounds, mesoporous additives, and obtained nanocoatings, selected and developed within Reliance project, is the overriding goal of work package 7 (WP7). These materials will be tested, considering a wide range of bacteria, fungi, and viruses, to include SARS-CoV-2. Furthermore, nanotoxicological study of the nanocoating developed will be addressed, including environment and in-vivo test (inhalation). The testing activities will ensure the effectiveness of the bioactive compounds, mesoporous additives, and obtained nanocoatings as well as their safety.
The activities in WP 7 are carried out by Reliance project partners Tor Vergata University, Policlinico Militare (Italian Ministry of Defence), Centexbel, Ineris, and Tekniker. During the first six-month period, they started to design the set-up for virus and bacteria analyses using the reference methods while also adding testing of some essential oils such as Carvacrol and Eugenol.
Carvacrol and Eugenol were initially tested to evaluate their antibacterial activities towards Escherichia coli (G-) and Bacillus clausii (G+). The Minimal Inhibitory Concentration (MIC) tests were performed by the broth microdilution method. The MIC is defined as the lowest concentration of the essential oil at which the bacteria does not demonstrate visible growth. The essential oils were firstly dissolved in an equal volume of Dimethyl sulfoxide (DMSO), then serial two-fold dilutions from 5.000%–0.002% (v/v) of the essential oils were prepared, and 200 μL of each dilution dispensed into a 48-well micro-titer plate. Each well was then inoculated with 200 μL of the bacterial suspension containing 105 CFU, and the micro-titer plate was incubated at 37 °C for 24 h. The preliminary experiments reported in Figure 1, highlighted Carvacrol MIC=0.08 % for B. clausii (Gram+) while a MIC=0.15 % is reported for Eugenol. In the case of E. coli (Gram-), Carvacrol MIC=0.04 %, and Eugenol MIC=0.08 % were observed, suggesting a higher inhibitory activity of Carvacrol vs. Eugenol for the tested bacteria.
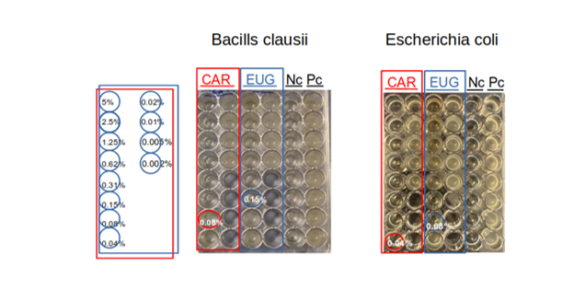
Fig.1
Antimicrobial activity evaluation by MIC for Carvacrol (CAR) and Eugenol (EUG) on B. clausii and E. coli. Nc, negative control (no bacteria), Pc, positive control (absence of EO).
To determine the mechanism of action of the selected essential oils on different viruses, the first experiments were carried out using chikungunya virus (CHIKV). CHIKV was isolated from a clinical sample by the Scientific Department of Policlinico Militare (Italian Ministry of Defence), and propagated in Vero cells. The viral titer was determined by plaque assay. In order to determine the virucidal activity of Carvacrol and Eugenol, equal volumes (0.5 ml) of CHIKV suspension, containing 10^5 PFU/mL, and essential oils were mixed with a final concentration of 0.5%, that resulted not toxic to the cells (Figure 2).
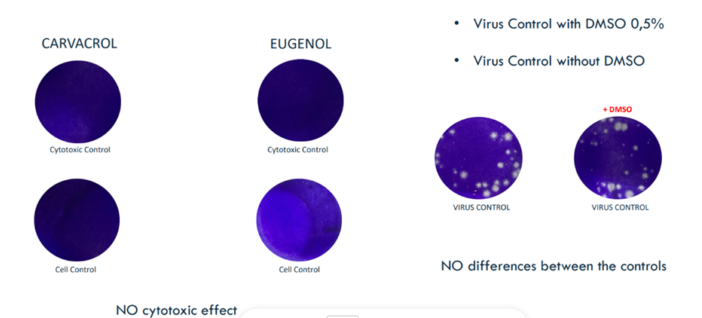
Figure 2. Study of cytotoxic effect and DMSO effect.
The effect of the essential oil addition at different time points (1, 3, 5, 15, 30, 45 and 60 min) was evaluated. As shown in Figure 3, viral inhibition by 50% was observed starting from 1- and 3-minutes treatment with Carvacrol and Eugenol, respectively. Interestingly, the antiviral activity of the compounds increased over time, reaching a percentage of inhibition of about 100 % upon 15 min incubation.
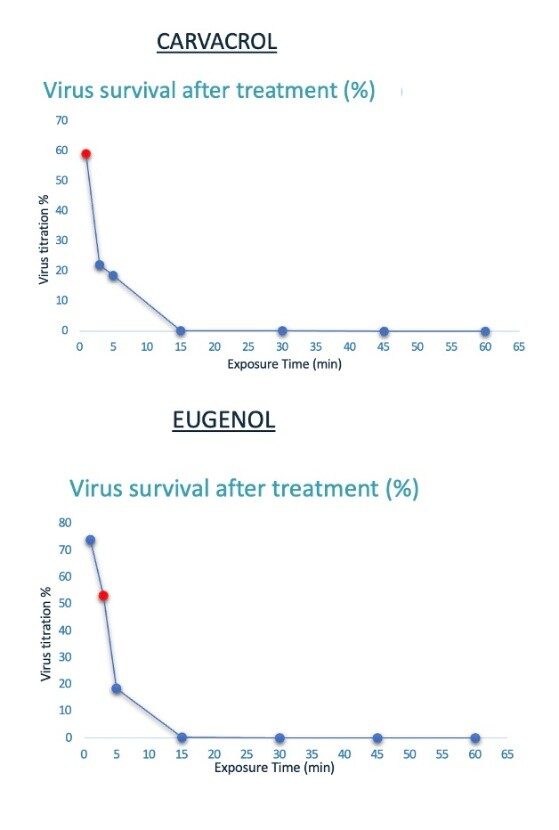
Figure 3. CHIKV survival after treatment over time

Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Health and Digital Executive Agency (HADEA). Neither the European Union nor the granting authority can be held responsible for them.
© All Rights Reserved 2025 | Reliance-HE
Designed and Developed by Europroject

