
January 12, 2026
A Safety-By-Design Approach for Human Hazard Evaluation of Bio-Based Self-Disinfecting Nano-Coatings
RELIANCE develops bio-based nanoparticle formulations with effective antimicrobial properties against a broad spectrum of pathogens, while ensuring safety for humans and the environment. The work activities within work package 7 of the project comprise a hazard evaluation to support the development of safe and sustainable products. Human hazard assessment is done through an oriented Nanotoxicology testing-based strategy, guided by a report of the regulatory requirements expected in respect of the developed formulations and product applications. Efforts are made for integrating new concepts and methodologies such as “One Substance One Assessment” and New Approach Methodologies (NAMs).
Methodology
Through a regulatory toxicology evaluation, partners from INERIS identified textile (including textiles for medical applications), food contact materials and potential worker safety (under REACH/OSHA) regulations to be prioritized when considering product formulations and applications. Based on these regulations, biocompatibility (ISO 10993), cytotoxicity, skin and eye irritation/sensitisation, inhalation exposure risk assessment and leaching (ISO 15181) are among the most of concern endpoints, in respect to potential human exposures. Genotoxicity may not be required since the individual compounds are not listed as genotoxic.
A testing strategy based on a safety-by-design approach with a tier 1 (in vitro) and a tier 2 (in vivo) was then chosen. Nanocoating components and the final formulations used for the manufacturing of the industrial products were both tested. Testing includes specific hazard categories such as skin and eye irritation (OECD TG 439,492), sensitization/allergy (DA 497, OECD TG 442), as well as potential inhalation hazard, including in vitro using Air liquid interface (ALI) exposures. These advanced in vitro methods have demonstrated good reliability for predicting the acute pulmonary toxicity of nanoparticles[1]). Hazard evaluation of final products such as treated fabrics is also performed (ISO 10993-5:2009). Potential risks of sub-chronic pulmonary toxicity, including toxicokinetic evaluation, is assessed using in vivo methods. In vivo experiments are conducted if long-term effects are suspected, as such effects (e.g., allergies) are particularly difficult to assess using NAMs in the case of particle exposures[2] (Wareing et al. 2024).
Results
Components and final formulations were tested. The bio-based self-disinfecting nano-coatings did not show any irritation for the skin or eye potential according to OECD TG 439 and 492. In vitro testing of lung alveolar cells in co-culture with macrophages both in submerged and at the ALI highlighted some mild cytotoxicity at high doses with SMIN, Cu-SMIN, Cu-SMIN-Carvacrol. More toxicity is observed when polyDMAEMA is added to the formulation for a controlled release of the essential oil carvacrol. Although, SMIN and Cu-SMIN did not induce any inflammation, addition of Carvacrol and poly-DMAEMA to the formulation did induce inflammation. Although some effects are observed on lung cells, treated fabrics with all the different components and formulations listed above did not induce any cytotoxicity (ISO 10993-5:2009). The testing strategy and preliminary results are summarized in the figure below (Figure 1).
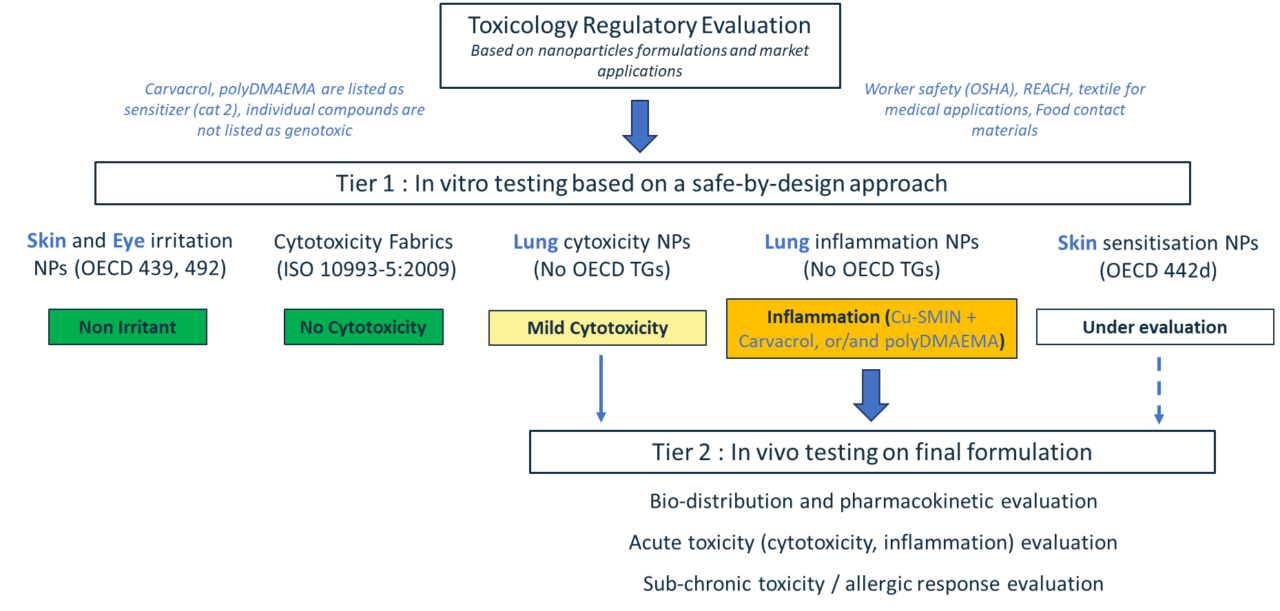
Figure 1. Nanotoxicology evaluation performed within RELIANCE.
Conclusion and Perspectives
The nanotoxicology evaluation is still ongoing. Based on the reported results, further testing is required to ensure product safety which includes sensitisation assessment in vitro and testing in vivo. These new results will be released shortly and will support a potential regulatory dossier for the usage of the developed Bio-Based Self-Disinfecting Nano-Coatings.
Contributor: INERIS
[1] Loret, T., et al. Predicting the in vivo pulmonary toxicity induced by acute exposure to poorly soluble nanomaterials by using advanced in vitro methods. Part Fibre Toxicol 15, 25 (2018). https://doi.org/10.1186/s12989-018-0260-6
[2] Pomar-Portillo, V., et al. "Methods and tools for the safety assessment part of the European Commission’s safe and sustainable by design framework when applied to advanced materials. Environ Int 109904 (2025). https://doi.org/10.1016/j.envint.2025.109904




Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or the European Health and Digital Executive Agency (HADEA). Neither the European Union nor the granting authority can be held responsible for them.
© All Rights Reserved 2025 | Reliance-HE
Designed and Developed by Europroject

